In September 2020, the World Health Organization (WHO) announced the EMERGENCY USE AUTHORISATION of two Antigen(Ag)-based rapid diagnostic tests (RDTs) by SD Biosensor and Abbott for COVID-19 testing.
Abbott panbio COVID-19 rapid test, Corona-Antigen test, nose swab
This diagnostic rapid test can be used as an aid in the diagnosis of SARS-CoV-2 infection.
- Quantity: 25 pcs/kit
- Fast identification of potentially contagious individuals
- Test results in 15 minutes
- Deploy at large scale at point of care
- Can be used in a wide variety of non-laboratory settings
- No special/additional instruments required
- Self-contained tube with “break off” swab minimizes staff exposure
- Extraction tube is fully enclosed for disposal
Manufacturer product reference: Abbott, 41FK10
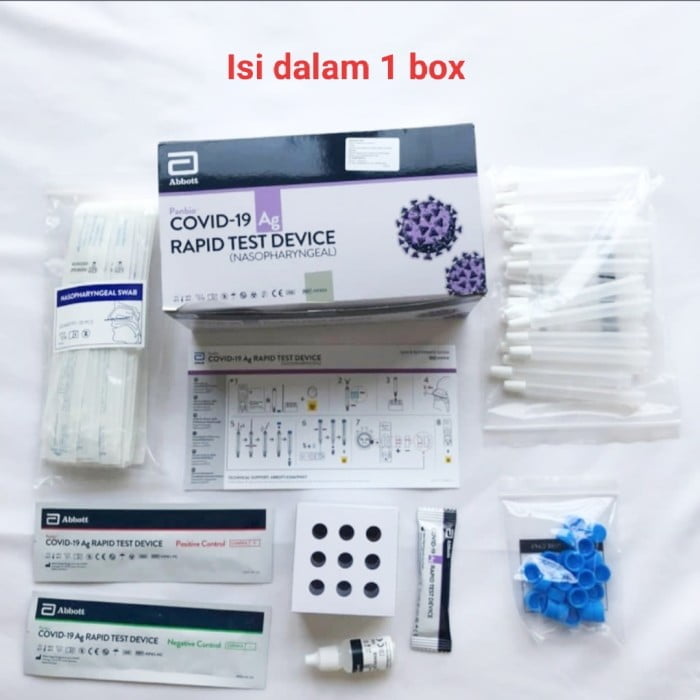
Items supplied with:
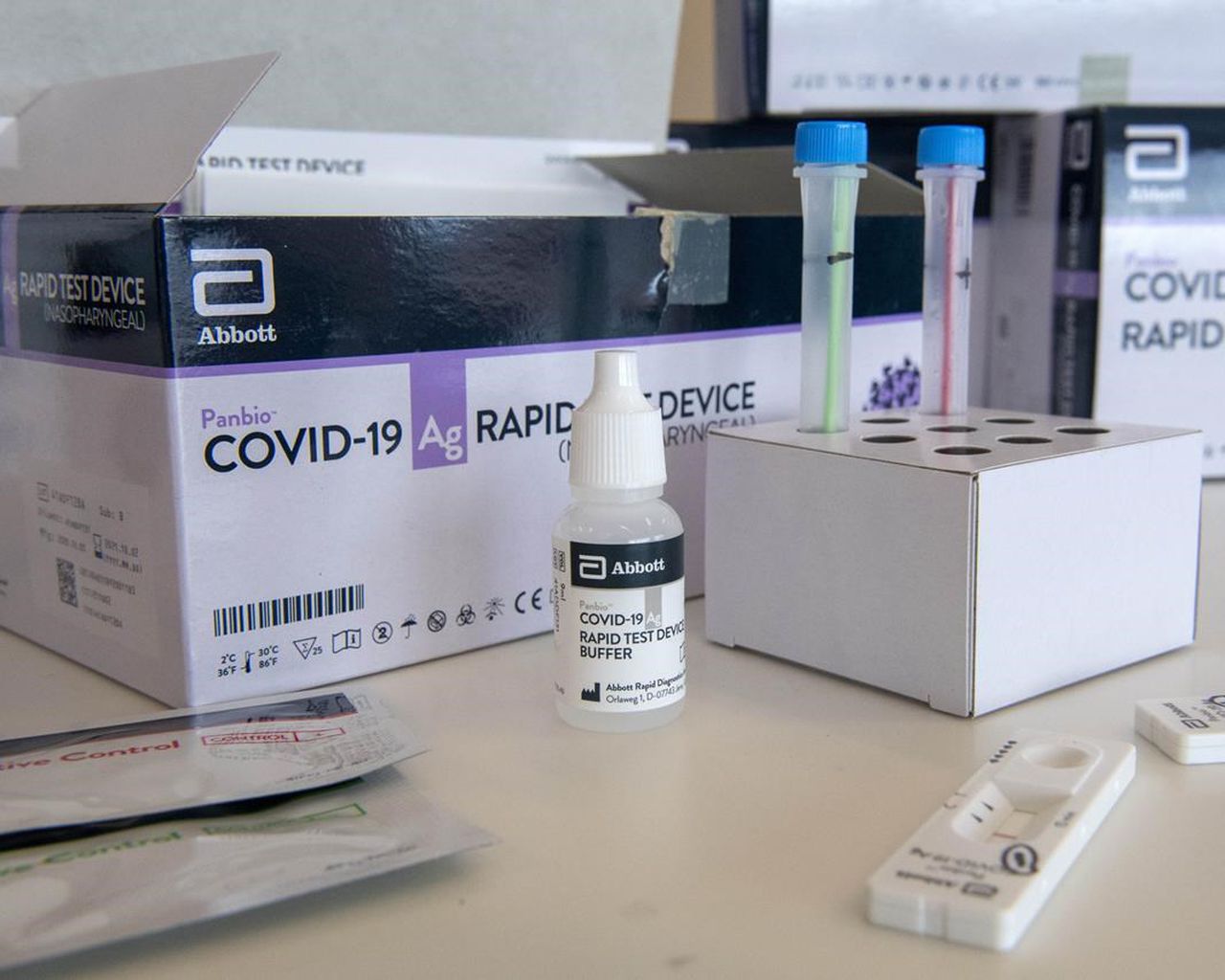
·25 x Test devices with desiccant in individual foil pouch
·1 x Buffer bottle (9 mL)
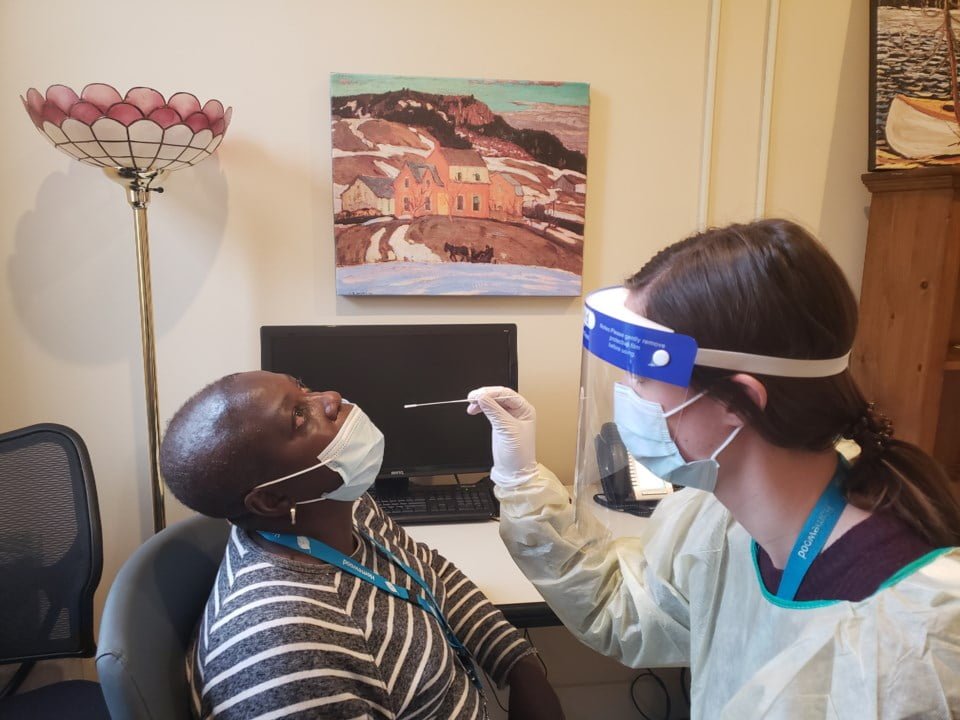
·25 x Sterilized nasopharyngeal swabs for sample collection
·25 x Extraction tubes
·25 x Extraction tube caps
·1 x Positive control swab
·1 x Negative control swab
·1 x Tube rack
·1 x Quick reference guide (Nasopharyngeal)
·1 x IFU
Materials required, but not Provided:
•Personal Protective Equipment, Timer, Biohazard container
Shelf life:
12 months from the date of manufacturing; do not use beyond its expiry date.
Weight & volume:
Estimated weight: 0.44 kg
Estimated volume: 4.312 dm3
Storage & transportation condition:
Must be stored at 2 – 30°C. Protect against direct sunlight. Do not freeze the kit. When stored in a refrigerator, all kit components must be brought to room temperature (15-30 °C) for a minimum of 30 minutes prior to performing the test.
Packaging & labelling:
Primary packaging: All contents of Panbio COVID-19 Ag Rapid Test Device, kit of 25 tests
Secondary packaging: One kit Panbio COVID-19 Ag Rapid Test Device, kit/25 tests
General recommendations:
·Do not re-use the test kit.
·Do not use the test kit if the pouch is damaged or the seal is broken.
·Do not mix reagents of different lots.
·Wear personal protective equipment, such as gloves and lab coats when handling kit reagents. Wash hands thoroughly after the tests are done.
Following WHO’s emergency use authorisation of two Antigen (Ag)-based Rapid Diagnostic Tests (RDTs) by SD Biosensor and Abbott, NCDC carried out a national validation. These two Ag-based RDTs have now been approved for use in congregate settings.
user manual for Abbott panbio COVID-19 rapid test