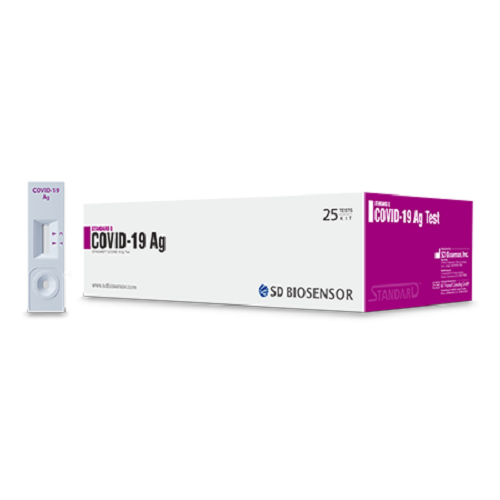
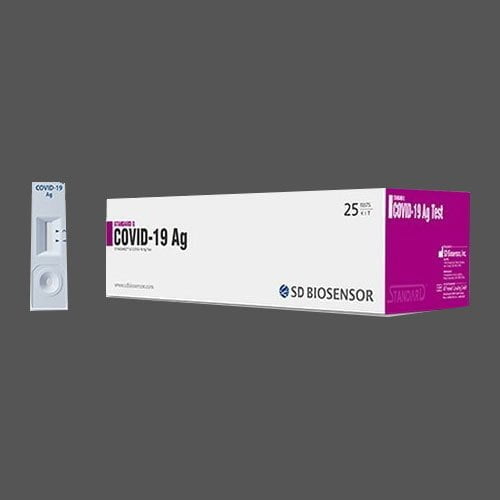
STANDARD Q COVID-19 Ag Test is a rapid chromatographic immunoassay for the qualitative detection of specific antigens of SARS-CoV-2 present in human nasopharyngeal specimens. This product is intended for healthcare professionals at the clinical setup and point of care sites, as an aid to early diagnosis of SARS-CoV-2 infection patient with clinical symptoms of SARS-CoV-2 infection. It provides only an initial screening test result. This product is strictly for medical professional use only and not intended for personal use. The administration of the test and the interpretation of the results should be done by a trained health professional. The result of this test should not be the sole basis for the diagnosis; confirmatory testing is required.
Coronavirus is a single-stranded positive-sense RNA virus with an envelope of about 80 to 120 nm in diameter. Its
genetic material is the largest of all RNA viruses and is an important pathogen of many domestic animals, pets,
and human diseases. It can cause a variety of acute and chronic diseases. Common signs of a person infected
with a coronavirus include respiratory symptoms, fever, cough, shortness of breath, and dyspnea. In more severe
cases, infection can cause pneumonia, severe acute respiratory syndrome, kidney failure, and even death. The
2019 new coronavirus, or “SARS-CoV-2 (COVID-19)”, was discovered because of Wuhan Viral Pneumonia cases
in 2019, and was named by the World Health Organization on January 12, 2020, confirming that it can cause
colds and the Middle East Respiratory Syndrome (MERS) and more serious diseases such as acute respiratory
syndrome (SARS). This kit is helpful for the auxiliary diagnosis of coronavirus infection. The test results are for
clinical reference only and cannot be used as a basis for confirming or excluding cases alone.
Intended use
STANDARD Q COVID-19 Ag Test is a rapid chromatographic immunoassay for the qualitative detection of specific
antigens to SARS-CoV-2 present in human nasopharynx. This test is for administration by healthcare workers and
labs only, as an aid to early diagnosis of SARS-CoV-2 infection in patient with clinical symptoms with SARS-CoV-2
infection. It provides only an initial screening test result. This product is strictly for medical professional use only
and not intended for personal use. The administration of the test and the interpretation of the results should
be done by a trained health professional. The result of this test should not be the sole basis for the diagnosis;
confirmatory testing is required.
Test principle
STANDARD Q COVID-19 Ag Test has two pre-coated lines, “C” Control line, “T” Test line on the surface of the
nitrocellulose membrane. Both the control line and test line in the result window are not visible before applying
any specimens. Mouse monoclonal anti-SARS-CoV-2 antibody is coated on the test line region and mouse
monoclonal anti-Chicken IgY antibody is coated on the control line region. Mouse monoclonal anti-SARS-CoV-2
antibody conjugated with color particles are used as detectors for SARS-CoV-2 antigen device. During the test,
SARS-CoV-2 antigen in the specimen interact with monoclonal anti-SARS-CoV-2 antibody conjugated with color
particles making antigen-antibody color particle complex. This complex migrates on the membrane via capillary
action until the test line, where it will be captured by the mouse monoclonal anti-SARS-CoV-2 antibody. A colored
test line would be visible in the result window if SARS-CoV-2 antigens are present in the specimen. The intensity
of colored test line will vary depending upon the amount of SARS-CoV-2 antigen present in the specimen. If SARSCoV-2 antigens are not present in the specimen, then no color appears in the test line. The control line is used for
procedural control, and should always appear if the test procedure is performed properly and the test reagents
of the control line are working.
Kit contents
① Test device (individually in a foil pouch with desiccant) ② Extraction buffer tube ③ Nozzle cap
④ Sterile swab ⑤ Instructions for use
KIT STORAGE AND STABILITY
Store the kit at 2-30°C / 36-86°F out of direct sunlight. Kit materials are stable until the expiration date printed on
the outer box. Do not freeze the kit.
Advantage
- Test time : In 15 ~ 30 minutes
- Easy and convenient testing process for professionals
- Effective in detecting the SARS-CoV-2 variant
- Suitable for Point of Care Testing
- No need for extra equipment